- Case Study
- Open access
- Published:
Malaria from hyperendemicity to elimination along international borders in Yunnan, China during 2003‒2020: a case study
Infectious Diseases of Poverty volume 11, Article number: 51 (2022)
Abstract
Background
Border malaria is one of the most intractable problems hindering malaria elimination worldwide. Movement of both the human population and anopheline mosquitoes infected with Plasmodium spp. can cause cross-border malaria transmission. The Yunnan border area was still hyperendemic for malaria in the early part of this century. The objective of this case study was to analyze the strategies, interventions and impacts of malaria control and elimination in the Yunnan border area.
Main text
A total of 10,349 malaria cases and 17.1 per 10,000 person-years of annual parasite incidence (API) were reported in the border area in 2003. Based on natural village-based stratification, integrated interventions, including mass drug administration for radical cures and preventive treatment, clinically presumptive treatment of all febrile patients for malaria and indoor residual spraying or dipping bed nets with insecticides were successfully carried out from 2003 to 2013. The overall API was reduced to 0.6 per 10,000 person-years by 2013, while effective cross-border collaboration interventions dramatically reduced the malaria burden in the neighbouring border areas of Myanmar. From 2014 forward, the comprehensive strategy, including universal coverage of surveillance to detect malaria cases, a rapid response to possible malaria cases and effective border collaboration with neighbouring areas, successfully eliminated malaria and prevented reintroduction of malaria transmission in the Yunnan border area.
Conclusions
In Yunnan malaria burden has successfully reduced by dynamically accurate stratification and comprehensive interventions; and then the region achieved elimination and prevented reintroduction of malaria transmission through intensive surveillance, rapid response and border collaboration. Other border areas should perform their own intervention trials to develop their own effective strategy.
Graphical Abstract
Background
The World Health Organization (WHO) certified China malaria-free status on June 30, 2021 [1, 2]. Yunnan Province in southwestern China shares 4060 km of border with Myanmar (1997 km), Laos (710 km) and Vietnam (1353 km). Yunnan is a unique province with malaria ecology and vector system similar to those of five other countries in the Great Mekong Sub region (GMS) [3]. Frequent migrants and anopheline mosquitoes infected with Plasmodium spp. crossing the border, and underdeveloped health services can lead to cross-border transmission of malaria parasites [4, 5]. These factors underline the original hyperendemicity in the Yunnan border area [6], and malaria elimination was truly difficult in the area. Malaria in the Yunnan border area definitely impaired its elimination in China [7]. Malaria elimination in China is a remarkable achievement and the culmination of seven decades of dedicated effort by the national malaria programme and its partners. Border malaria elimination in Yunnan has strongly contributed to this remarkable achievement [8]. Currently, Malaria is a continuous public health problem worldwide. Due to health service disruptions during the coronavirus disease 2019 (COVID-19) pandemic, there were an estimated 241 million malaria cases in 2020, increased from 227 million in 2019, and malaria deaths increased by 12% compared with 2019, to an estimated 627 thousand [9]. Border collaboration has promoted malaria elimination in the Yunnan border area [10]. Under the context of the COVID-19 pandemic, border collaboration for malaria control activities is limited when border crossings are strictly limited. The surveillance data of the cross-border joint prevention and control project of malaria and dengue fever in Yunnan of China and GMS showed malaria resurgence in part of the border area of neighboring countries. For example, the Laiza and nearby areas in Kachin Special Region II (KR2) of Myanmar reported 274 malaria cases in 2019 followed by 1587 cases in 2020. The resurgence of malaria in some border areas of neighboring countries suggests that China should prepare well to respond to the reintroduction of malaria transmission in the Yunnan border area for the post COVID-19 era. The objective of this case study was (1) to analyze the strategies and interventions used from malaria control to its elimination and their impact during 2003‒2020, and (2) to present a strategy of preventing the reintroduction of malaria transmission in the Yunnan border area.
Methods
Study site
The border county is defined as the border area in this study. There are 25 border counties in Yunnan, namely 17 counties bordering Myanmar, one (Mengla) with Myanmar and Laos, one (Jiangcheng) with Laos and Vietnam, and six counties with Vietnam. The Yunnan border area has a tropical or subtropical monsoon climate and is populated by 9,093,082 people in 2020. A hot climate, adequate precipitation and forests provide a suitable environment for the growth and reproduction of mosquitoes and for malaria transmission. With a complex vector community, Anopheles minimus and An. sinensis were identified as the primary and secondary vectors of malaria in this area [11, 12]. Year-round malaria transmission occurred in most parts of the border area prior to elimination. All four of the parasite species (i.e., P. falciparum, P. vivax, P. malariae and P. ovale) were detected in the area [13]. There were no natural or artificial barriers along the boundary prior to the COVID-19 pandemic. Thirteen indigenous ethnic minorities live across the boundary. The border area is an underdeveloped area with poor communities, marginalized populations and weak health services. The border areas of the three neighboring countries present civil unrest (mainly in Myanmar), unpermitted border crossers and a high malaria burden [14]. Each of these factors challenged the feasibility of border malaria elimination in the Yunnan border area.
Data sources and collection
To collect data on malaria cases, intervention activities and control strategies, all available paper-based records related to border malaria surveillance and interventions from 2003 to 2020 were reviewed at the Yunnan Institute of Parasitic Diseases (YIPD). As the Chinese Information System for Disease Control and Prevention (CISDCP) began to cover all Yunnan’s counties since 2008 [15, 16]; therefore, the relative data during 2008–2020 were obtained from the CISDCP. In addition, all available documents and literature about the border malaria situation and control activities in Yunnan and neighboring countries (Vietnam, Laos and Myanmar) were also reviewed. These studies and documents include original work records, books, annals, guidelines and operational manuals about malaria control and elimination in Yunnan.
Data analysis
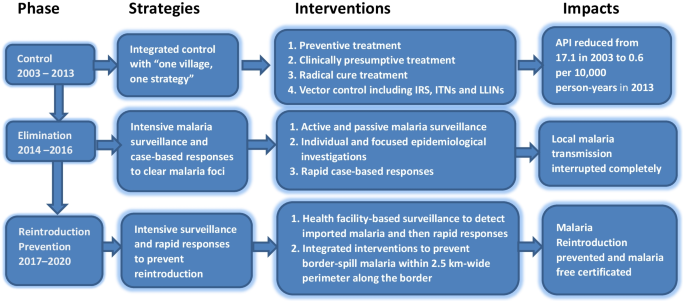
To analyse and present the data, the malaria programme from hyperendemicity to elimination in the border area during 2003‒2020 was divided into three phases, namely, control phase (2003‒2013), elimination phase (2014‒2016) and reintroduction prevention phase (2017‒2020) (Fig. 1). This phase division was based on the WHO’s recommendation on malaria programme phases and milestones on the path to malaria elimination [17] and the local context in Yunnan Province. The control phase was the period with an overall annual parasite incidence (API) ≥ 1.0 per 10,000 person-years, the elimination phase was the period with API < 1.0 per 10,000 person-years but with indigenous malaria cases, and the reintroduction prevention phase was the period from local interruption of malaria transmission forward.
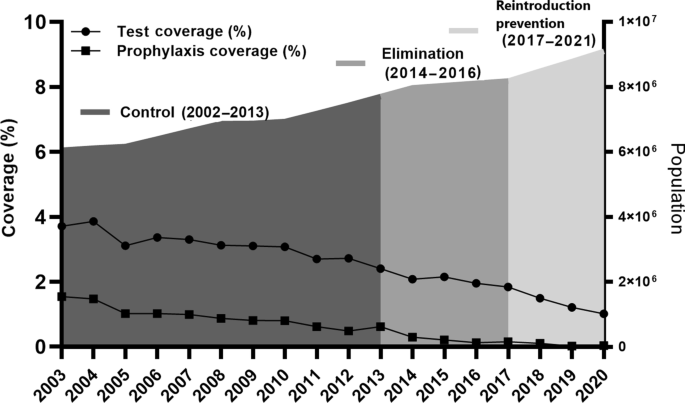
The key events that were considered having significant impact on malaria control and elimination in the Yunnan border area were summarized to list in Table 1. For each phase, the strategies and interventions were described, including stratification of malaria areas, treatment of malaria cases, vector control, surveillance and focus responses from malaria hyperendemicity to elimination. To present the malaria case surveillance and drug-based prevention, annual coverage of laboratory tests for malaria and preventive treatment were calculated for each year of the three phases.
Drug-based treatment is the primary intervention to clear malaria parasite reservoirs and interrupt transmission [18]. To solve the challenges of asymptomatic and submicroscopic parasite density (especially for P. vivax), and the limitations of microscopist ability and rapid diagnostic tests (RDTs), an expanded treatment strategy was used during the control phase. Ratios of the number of laboratory-confirmed malaria cases versus the number of people treated with antimalarial drugs were calculated.
To present the impact of these strategies and interventions, the API of the overall border area was calculated for each year of 2003–2013. When local transmission was interrupted, malaria was mainly imported from endemic areas of other countries, and calculation of the API was not appropriate [17]. Only the number of imported malaria cases detected and their infection sources were counted since 2014. The years of local certification of malaria free for eight border prefectures were used to document the impact of elimination interventions.
Results
Control phase from 2003 to 2013
Integrated control strategies of “one village, one strategy”
Facing hyperendemicity in this early century, the approach of “one village, one strategy” that was developed and started in Yunnan in the early 1990s, and continuously carried out during 2003‒2013. This strategy categorized all natural villages into four types each year dynamically according to their malaria incidence in the last 3 years. Type I was villages with API ≥ 1%, or malaria clinical attack rate (proportion of people who had clinical symptoms of malaria among all residents in the village) in last year ≥ 10%; Type II was villages with API < 1%, or malaria clinical attack rate < 10% in last year, but with indigenous cases in the last 3 years; Type III was villages without indigenous cases, only with imported cases in the last 3 years; and Type IV was villages without any malaria cases in the last 3 years (Additional file 1: Table S1) [19].
Border collaboration and funding application
Cross border collaboration was initiated to reduce malaria burden in the border areas of neighbouring countries during this phase. The former Ministry of Health of China and the Ministry of Health and Sports of Myanmar signed “The Agreement of Cross Border Malaria Control” on June 7, 2005 [20]. “The joint malaria control project along the China–Myanmar Border” has been carried out since 2005 [21]. Under the agreement framework, YIPD and Health Poverty Action successfully applied for and carried out the sixth and tenth rounds of the Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM) with two malaria projects conducted along the China–Myanmar border from 2007 to 2013 [15].
Natural village-based stratification and interventions
To solve the problems of high morbidity, specificity and complexity, the strategy of natural village-based stratification and interventions was continuously conducted in the border area. Mass drug administration for radical cure treatment was conducted in type I villages in the low transmission season (December–February of next year) and for preventive treatment in the high transmission season (May‒October). Radical cure treatment was only administered to people with a malaria attack history in the last 2 years in type II‒IV villages [19, 22]. With a decreasing malaria incidence, Fig. 2 indicates that the coverage of preventive treatment, namely, the percentage of people with at least one drug administration for prophylaxis, decreased from 1.5% in 2003 to 0.6% in 2013 (Additional file 1: Table S2). To accelerate the Yunnan pace of malaria elimination, radical cure treatments were expanded to clear parasite reservoirs as soon as possible. The ratio of the number of people with radical cure treatment versus the number of laboratory confirmed malaria cases increased from 3.2 in 2003 to 17.3 in 2010, followed by reduction to 5.1 in 2013 (Table 2). Meanwhile, indoor residual spraying (IRS), insecticide-treated bed nets (ITNs) with pyrethroid or delivering long lasting insecticidal bed nets were conducted in type I and II villages. IRS with pyrethroid insecticides was only carried out in houses of malaria patients and their neighbours in type III and IV villages [19].
Impacts on malaria burden
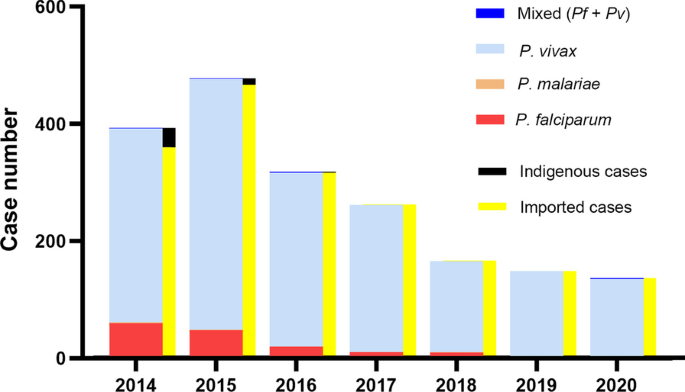
In 2003, a total of 10,349 cases and 17.1 per 10,000 person-years of API were reported in the Yunnan border area (Fig. 3). The number accounted for 67.1% of 15,431 confirmed malaria cases across Yunnan Province. A survey found that more than 90.0% malaria cases were underreported in the border area in 2002. This underreported rate was higher than the mean underreported rate (88.8%) in Yunnan [23]. Based on this survey of underreported malaria cases, it was estimated that there were approximately 100 thousand malaria cases in the border area in 2003. As a result of the intensive interventions, the API was successfully reduced to 13.5 per 10,000 person-years in 2006, followed by 2.3 per 10,000 person-years in 2010 and then 0.6 per 10,000 person-years in 2013 (Fig. 3). The dramatic reduction in malaria burden was also attributable to effective border collaboration for malaria control between China and Myanmar. The two GFATM projects successfully reduced the malaria burden by 90% in five Special Regions of Myanmar as well as by 95% in the Yunnan border area along the China–Myanmar border. The data on control activities and their impact on malaria burden were presented in detail in previously publised papers [10, 15]. The significant reduction in malaria cases made it possible to completely switch the malaria programme from control to elimination in Yunnan.
Elimination phase from 2014 to 2016
Strategies of “clearing malaria foci, tracking infectious sources”
Yunnan’s 104 inland counties kept pace with the country to start malaria elimination action since 2010. Due to the higher endemicity in the Yunnan border area, malaria elimination action was actually launched in 2014. Malaria elimination requires a universal coverage of malaria surveillance and a rapid response to any suspected malaria foci [17]. The Chinese “1-3-7” strategy requests reporting malaria cases within 1 day, confirmation and investigation of malaria cases within 3 days, and an appropriate public health response to prevent further transmission within 7 days [24]. The WHO recommends that the elimination phase starts in a district where the first program reorientation has been achieved; and where health facility data show an API < 1 per 1000 person-years at risk, equal to less than 100 new cases per year in a district with a population of 100,000 people [17]. In China, the smallest unit for elimination is a county, and most counties have a population of over 1 million. The national malaria elimination program therefore recommended that the elimination phase was initiated after achieving an API < 1 per 10,000 person-years. The national standards of county stratification for malaria elimination categorized all counties into four tiers, namely, type I with the presence of confirmed local case (s) in the last 3 years, with at least 1 year having an API ≥ 1 per 10,000 person-years; type II with the presence of confirmed local case(s) in the last three years, with an API < 1 per 10,000 person-years; type III without any local cases for at least 3 years, only imported cases; and type IV without a history of any local cases, only imported cases [25]. Following the national stratification standards for malaria elimination, Yunnan categorized its 129 counties into three tiers (no type IV), namely, 19 type I counties with 17 border counties, 55 type II counties with eight border counties, and 55 type III counties in 2010. According to the stratification, every county took malaria elimination as one of the governmental work objectives to establish a leadership and technical steering team. The strategy of “clearing malaria foci (parasite reservoirs), tracking infectious sources” were conducted by intensive surveillances, epidemiological investigations and rapid public health responses.
Interventions for intensive surveillance and rapid response
Following “The Protocol of Yunnan Malaria Elimination Action Plan (2010‒2020)”, the interventions of intensive surveillance and rapid response were conducted [26]. A total of 481,772 febrile patents were tested by microscopy or RDTs for malaria in the border area from 2014 to 2016 (Fig. 2, Additional file 1: Table S2). Following the “1-3-7” work approaches, all 1240 malaria cases detected were reported within 1 day; individual epidemiological surveys were completed within 3 days; and focused epidemiological investigations and public health responses were conducted within 7 days [24]. Strengthened malaria surveillance ensured the timely detection of parasite infections and rapid responses to clear parasite reservoirs for preventing further transmission.
Impacts on malaria transmission
The Action Plan of China Malaria Elimination 2010–2020 scheduled reducing the API to less than 1 per 10,000 person-years in each county of the Yunnan border by the end of 2015. This goal was actually achieved by 2013 with a mean API of 0.6 per 10,000 person-years (Fig. 3), except Tengchong with an API of 2.0 per 10,000 person-years due to imported malaria cases being included in the API and imported cases accounting for more than 95% of the total cases in Tengchong County (Additional file 1: Table S5). The WHO guidelines for malaria elimination do not recommend the inclusion of imported malaria cases in the calculation of API [17]. At last, the transmission of falciparum malaria has successfully been interrupted since the last locally falciparum malaria case was reported from Cangyuan County in May 2015, and then vivax malaria transmission has finally been interrupted since the last locally vivax malaria case was reported from Yingjiang County on April 17, 2016 (Fig. 4) [27].
Reintroduction prevention phase from 2017 to 2020
Strategies of timely malaria detection and response
The WHO malaria elimination certification standard is that the chain of indigenous malaria transmission by Anopheles mosquitoes has been interrupted nationwide for at least the past 3 consecutive years, and a country must also demonstrate the capacity to prevent reintroduction [28]. However, Yunnan borders three malaria endemic countries. Imported malaria can be caused by both border crossers and parasite-infected Anopheles mosquitoes, which fly over the boundary from endemic areas of neighbouring countries [10]. After the interruption of malaria transmission, the national stratification standards of malaria elimination were no longer for the actual situation in the Yunnan border area. To effectively prevent the reintroduction of malaria transmission, Yunnan further categorized 25 border counties into three tiers (types A, B and C) based on the malaria hyperendemicity in border area of neighbouring countries and the specificity of 25 border counties in 2017. The type A and B counties are to border with Myanmar or/and Laos. The type A counties are with 10 and more imported malaria cases from the border areas of neighbouring countries or with malaria cases lacking of travel history in the endemic areas of other countries during 2015‒2016. The type B counties are with less than 10 imported malaria cases from the border area of neighboring countries and the imported malaria cases with clear travel history in the endemic areas of other countries during 2015‒2016. The type C counties are only to border with Vietnam. The 12 type A counties needed more input of human and financial resources to carry out more intensive interventions, including vector control. Seven type B counties needed to strengthen malaria case surveillance. The six type C counties bordering Vietnam did not need additional investment or special interventions. The results of 291 Anopheles mosquito mark-release-recapture experiments in 143 localities around the world estimated that the mean distance travelled of female Anopheles was not more than 2.5 km [29]. An assessment of the receptivity and vulnerability was conducted for each community within 2.5 km-wide perimeter border areas of Myanmar along the boundary. The assessment result proposed a total of 16 natural villages in the threat of border-spill malaria in 2018 (Additional file 1: Table S7). Border-spill malaria is defined as a kind of imported malaria that is caused by parasite-infected Anopheles from the border endemic areas of neighbouring countries.
Health facility-based surveillance and border-spill malaria prevention
For each of the border counties, passive detection was consolidated into normal health service. Health services personnel were trained to remain vigilant to ensure universal coverage of malaria detection and react promptly to any suspected malaria cases. The unpermitted travellers cross borders frequently and present in frontier townships. With assistance from villager leaders and health workers to monitor cross border travellers, and refer febrile patients to the township hospitals for malaria test, community-based malaria detection and screening of migrants and travellers were carried out in frontier townships. To prevent the border-spill malaria, integrated interventions that include proactive and passive detection of the malaria parasites, enhancement and optimization of vector surveillance, further strengthening of timely detection with high-quality confirmed diagnosis and prompt action based on the surveillance results were carried out in these 16 high-risk reintroduction villages [10]. These interventions ensured universal coverage of malaria surveillance to detect malaria cases and timely public health responses in the Yunnan border area.
Impacts on malaria free certification
The threat from Vietnam and Lao PDR is slight. The overall incidence of malaria is low in Vietnam, with malaria transmission being interrupted in northern Vietnam [30]. Malaria control has also made rapid progress toward localized elimination goals in the northern provinces of Laos [31]. Yunnan first achieved malaria free status for at least 3 years in Honghe Prefecture with three counties bordering Vietnam only in 2015 [16], and then Wenshan Prefecture, with three counties bordering Vietnam in 2016. Beginning from the Honghe Prefecture, the eight border prefectures and their 25 border counties were gradually evaluated and certified for malaria free by Yunnan itself following the national standards of malaria elimination assessment (Table 3). The intensive interventions effectively prevented the reintroduction of malaria transmission to ensure timely national and WHO malaria-free certification. The China National Health Commission finally assessed and certificated Yunnan malaria free in June 2020. Experts of the WHO Malaria Elimination Certification Panel (MECP) visited two border counties, Menglian and Yingjiang, to conduct field assessment for China’s national malaria elimination certification in May 2021. The experts of the WHO MECP highly appreciated the infrastructure and equipment, the competence of the staff of the health system and supporting organization, the data management and the record system during their visits.
Discussion
Malaria elimination in the international border areas is one of the challenges that countries face today in their path to malaria elimination. Interruption of malaria transmission and continuous maintenance of malaria free in the Yunnan border area allowed the WHO’s certification of malaria elimination for China [2, 7]. This case study presented the story of malaria from hyperendemicity to elimination in the Yunnan border area. The following experiences and lessons can be learned from this case study.
Experiences
Universal coverage of malaria surveillance
The WHO certification of malaria elimination requires applicant countries to provide evidence that (1) local malaria transmission has been fully interrupted, resulting in zero indigenous human malaria cases for at least the past 3 consecutive years (36 months), and (2) an adequate program for preventing reintroduction of malaria transmission is fully functional throughout the country [17, 28]. The “1-3-7” approach of malaria elimination [24] can only be performed after malaria cases are detected. Finding malaria cases in time is the prerequisite of using the “1-3-7” approach to interrupt and prevent further transmission. To ensure the sensitivity of malaria surveillance, a surveillance system of malaria cases in the border area has gradually achieved universal coverage in the elimination stage, which includes proactive and passive case detection, community-based malaria detection and screening of migrants and travellers in frontier townships. Due to few malaria cases during the elimination stage, malaria diagnosis and treatment can no longer be a money-making channel. Based on the local governmental health policy, private sector, village leaders and village health workers help to monitor migrants and refer febrile patients to perform tests for malaria in health institutions with laboratory test. Remote villages have trained health or malaria workers who can use RDTs to test febrile patients for malaria [10].
Accurate and dynamically adjusted stratification
The WHO recommends that stratification should be initially performed at the lowest geographical level for which operational decisions can be made [17]. In the 1990s, Yunnan developed natural village-based stratification to perform cost-effective interventions, and the stratification and intervention measures were adjusted every year [19]. Appropriate investment made it possible to fully carry out natural village-based stratification and interventions from 2003 to 2013. The integrated interventions dramatically reduced the malaria burden. The WHO also recommends that interventions are expected to change the epidemiology of malaria rapidly and profoundly, and the stratification of malaria maps should be revised frequently. As transmission intensity is progressively reduced, stratification needs to include vulnerability and receptivity to malaria [17].
“The Action Plan of China Malaria Elimination (2010–2020)” defined a county as a unit of elimination. The 25 border counties were categorized into two tiers according to the national stratification standards for malaria elimination, namely 17 type I counties and eight type II counties [25, 26]. Yunnan interrupted malaria transmission in 2017, and the national stratification standards for malaria elimination were no longer suitable for the actual situation. Yunnan stratified the 25 border counties into three types (A, B and C) in 2017 and then identified 16 natural villages with high risks of border-spill malaria in 2018 (Additional file 1) to guide resource allocation and the use of a more targeted strategy.
Based on the experiences of malaria control from hyperendemicity to elimination, Yunnan designed the “3 + 1” strategy in 2019 to prevent reintroduction of malaria transmission, namely, (1) comprehensive and intensive malaria interventions in the area within a 2.5 km wide perimeter along the international border to prevent border-spill malaria, (2) community-based malaria surveillance to identify international migrants with possible malaria in the frontier townships, (3) consolidate surveillance into normal health services to maintain vigilance of health personnel to malaria signs, and + 1) emphasize the need to strengthen collaboration with neighboring countries to reduce their malaria burden with a clear focus on border areas with China [10]. The “3 + 1” strategy is in accordance with the principle of the WHO recommended malaria elimination strategy [17].
Clearing parasite reservoirs
A comprehensive malaria control strategy includes clearing parasites with antimalarial treatments, interrupting transmission by vector control and protecting vulnerable individuals. Drug-based treatment is the primary intervention in malaria control and elimination, and clearing parasites with antimalarial drugs is the most direct and effective approach. Asymptomatic and submicroscopic parasite density, especially for P. vivax, and limitations of microscopist ability and RDTs may lead to underdetection or misdiagnosis [18, 22]. To clear parasite reservoirs for the reduction of malaria infectious sources, expanded clinical and radical cure treatments were conducted in highly endemic years in the border area. The expanded clinical treatment is that the treatment includes both lab confirmed cases and suspected malaria cases in health facilities. The expanded radical cure treatment is that treatment includes people with both history of lab confirmed malaria and suspected malaria in the last 2 years. The ratios of clinical and radical cure treatment to laboratory-confirmed malaria cases were approximately three during 2003‒2006. To accelerate the malaria elimination process, the ratio of radical cure treatment versus laboratory-confirmed malaria cases reached 17.3 in 2010 due to the expanded radical cure treatment (Table 2). Based on these experiences and results of the intervention trial in Cambodia [32], mass drug administration can rapidly reduce the malaria burden in hyperendemic areas; however, it might not be necessary for mesoendemic situations. When malaria endemicity is still high, treatment for all confirmed, clinical and suspected cases, not just targeting confirmed malaria cases, might be necessary [18, 22]. After parasite reservoirs cleared, clinically presumptive treatment of suspected cases is not recommended again. Confirmatory diagnosis for treatment with antimalarial drugs is recommended and practiced because of a few of malaria cases and the high accessibility of laboratory malaria diagnosis for people in the border area. The high accessibility of laboratory test for malaria is assured by the improvement of the laboratory test capacity in public health facilities and the locally improved transportation for residents.
Comprehensive interventions
A systematic network literature review compared malaria prevention measures, including ITNs including long lasting insecticidal bed nets and insecticidal-treated bed nets, IRS, prophylactic drugs (PD) and untreated nets (UN), against no intervention. The study demonstrated that only ITN [rate ratio (RR): 0.5, 95% CI: 0.3–0.7] showed preventive efficacy precision while other methods, PD (RR: 0.2, 95% CI: 0.004–15.4), IRS (RR: 0.6, 95% CI: 0.2–1.6) and UN (RR: 0.7, 95% CI: 0.3–1.9), indicated considerable uncertainty associated with their point estimates [33]. The results of the review document that no single preventive measures can certainly prevent malaria. An analysis of simulated trial data using a transmission model also documents that a longer duration of prophylaxis leads to a greater measured efficacy of radical cure treatment for P. vivax, particularly at higher transmission intensities [34]. The results of this study indicate that integrated interventions are more effective than a single measure.
To control and eliminate malaria, integrated interventions, including proactive and passive case detection, vector surveillance and evidence-based vector control and preventive treatment with drugs, have been used in the border area. In the border area, approximately 100 thousand people received prophylactic drugs for prevention in 2003, and then approximately 2500 people in border communities that neighbouring with the hyperendemic areas of Myanmar received prophylactic drugs to prevent border-spill malaria in 2020. Because of lacking the powerful data on border-spill malaria caused by anopheline mosquitoes infected with malaria parasites, the WHO just recommends prophylactic drugs for travellers in malaria endemic countries, not in the setting of malaria elimination [17, 28]. There is a viewpoint that prophylactic drugs should no longer be used in the phase of malaria elimination in China. However, when vector control measures cannot effectively prevent border-spill malaria, the intervention of prophylactic drugs is still needed for people residing in communities bordering the hyperendemic areas of neighboring countries [10] as well as travellers who want to go to endemic countries [22].
Lessons
Reduced collaboration increased the risk of malaria reintroduction
Communication and collaborative activities were significantly reduced after China’s GFATM malaria project was terminated in 2014. A slight malaria resurgence has appeared in some border areas of Myanmar since 2014 [18, 35]. The number of imported malaria cases correspondingly increased from 358 in 2013 to 594 cases in 2015 in Yunnan. The Laiza and nearby areas of KR2 with a population of approximately 30 thousand persons, are one of the malaria hotspot areas in the border area of Myanmar [10]. The number of reported malaria cases increased from 518 in 2013 to 2367 in 2016. The strengthened collaborative interventions between China and Myanmar during 2017‒2019 reduced the number of malaria cases to 274 in 2019. However, reduced collaborative interventions due to the COVID-19 pandemic led to malaria resurgence again, and a total of 1532 cases were reported in Laiza and nearby areas of KR2 from January to November 2021. The example of Laiza and nearby areas documents that reduced communication and collaboration may increase malaria incidence in the border areas of neighbouring countries and increase the risk of malaria reintroduction in China. In contrast, a reduction in malaria burden in the border area of neighbouring countries can help decrease the threat of malaria importation and reintroduction.
Maintaining vigilance of health personnel
Vigilance of health personnel, especially clinical doctors in hospitals, is critical to reduce imported malaria death and prevent reintroduction of malaria transmission in elimination settings [17, 28]. Under the current technical and transportation conditions in China, travelers from malaria-endemic countries can always obtain laboratory tests for malaria in time as long as clinical doctors recognize the necessity of test. In fact, a number of imported malaria deaths are mainly attributable to the delayed diagnosis of malaria because of clinical doctors losing their vigilance. For example, in November 2021, a Burmese patient with kidney failure was hospitalized in a county hospital in the border area. His resident doctor did not recognize the necessity of malaria testing for more than 3 weeks because of the lack of vigilance for malaria. The patient had to be moved to a high-level hospital due to his worsened condition, and then the high-level hospital tested him with P. malariae, which was one of the reasons for his kidney failure. Reducing vigilance and technical capacity in malaria diagnosis and treatment due to rarely seeing malaria patients anymore is therefore one of the challenges to prevent the reintroduction of malaria transmission in elimination settings [10].
Challenges in the context of the COVID-19 pandemic
The Yunnan border area is also one of the areas facing a high risk of the COVID-19 pandemic in China. To fight the COVID-19 pandemic, some human and financial resources were moved from malaria control to the response to the COVID-19 pandemic. In July 2021, when Yunnan tried to communicate with the Health Authority of Myanmar KR2 to collaborate in rolling back the resurgence of malaria, the KR2 Department of Health responded that they were too busy responding to the COVID-19 pandemic to have human resources fighting malaria. Although the border crossing is strictly limited under the context of the COVID-19 pandemic in Yunnan, the increased malaria incidence in the KR2 has led to malaria spilling over the boundary by Anopheles mosquitoes into communities in the Yunnan border area. From January to November 2021, Yingjiang County reported a total of 70 cases, and 63 of them were categorized into border-spill malaria cases. In the context of the COVID-19 pandemic and border collaboration limitations for malaria, comprehensive intervention, including proactive and passive case detection, vector surveillance, evidence-based vector control and preventative treatment with antimalarial drugs, should be undertaken to prevent border-spill malaria within a 2.5 km-wide perimeter along the boundary in Yunnan [10].
Conclusions
Malaria from hyperendemicity to elimination in the Yunnan border area can be attributed to governmental commitment, comprehensively effective interventions and collaboration with neighbouring countries based on the local context. Although malaria has been eliminated, and reintroduction of malaria transmission has been prevented, malaria importation from the endemic areas of neighbouring countries is still a continuous threat. Comprehensive interventions are continuously essential in preventing the reintroduction of malaria transmission. Access to technical measures requires strong governmental and social support. Other border areas should perform their own intervention trials to develop their own effective strategy of malaria control and elimination in the context of the governing system, malaria burden, health service structure, socioeconomic development and ecology. It can be helpful to refer to and adopt the experiences and lessons from this paper in their own malaria elimination program.
Availability of data and materials
Not applicable.
Abbreviations
- API:
-
Annual parasite incidence
- WHO:
-
World Health Organization
- GMS:
-
Greater Mekong Subregion
- COVID-19:
-
Coronavirus disease 2019
- YIPD:
-
Yunnan Institute of Parasitic Diseases
- CISDCP:
-
Chinese Information System for Disease Control and Prevention
- RDTs:
-
Rapid diagnostic tests
- IRS:
-
Indoor residual spraying
- GFATM:
-
Global Fund to Fight AIDS, Tuberculosis and Malaria
- CDC:
-
Centre for Disease Control and Prevention
- KR2:
-
Kachin Special Region II
- MECP:
-
Malaria Elimination Certification Panel
- ITNs:
-
Insecticide treated bed nets
- RR:
-
Rate ratio
References
Burki T. Triumph in China as it is certified malaria-free by WHO. Lancet Infect Dis. 2021;21:1220–1.
Cao J, Newby G, Cotter C, Hsiang M, Larson E, Tatarsky A, et al. Achieving malaria elimination in China 2021. Lancet Public Health. 2021;6:e871-872.
Cui L, Yan G, Sattabongkot J, Cao Y, Chen B, Chen X, et al. Malaria in the Greater Mekong Subregion: heterogeneity and complexity. Acta Trop. 2012;121:227–39.
Lai S, Sun J, Ruktanonchai N, Zhou S, Yu J, Routledge I, et al. Changing epidemiology and challenges of malaria in China towards elimination. Malar J. 2019;18:107.
Wang D, Li S, Cheng Z, Xiao N, Cotter C, Hwang J, et al. Transmission risk from imported Plasmodium vivax malaria in the China–Myanmar Border Region. Emerg Infect Dis. 2015;21(10):1861–4.
Xu J, Liu H. Border malaria in Yunnan, China. Southeast Asian J Trop Med Public Health. 1997;28:456–9.
Xu J, Liu H. The challenges of malaria elimination in Yunnan province, People’s republic of china. Southeast Asian J Trop Med Public Health. 2012;43:819–24.
Ministry of Health of the People’s Republic of China. Action plan of China malaria elimination (2010–2020). 2012. http://www.gov.cn/gzdt/att/att/site1/20100526/001e3741a2cc0d67233801.doc. Accessed 15 Oct 2013.
World Health Organization. World malaria report 2020. Geneva: World Health Organization; 2021.
Xu JW, Lee R, Lin ZR, Zhou YW, Shen HM, Zhou HN, et al. Intensive surveillance, rapid response and border collaboration for malaria elimination: China Yunnan’s ‘“3+1”’ strategy. Malar J. 2021;20:396.
Hii J, Rueda L. Malaria vectors in the Greater Mekong Subregion: Overview of malaria vectors and remaining challenges. Southeast Asian J Trop Med Public Health. 2013;44(Suppl 1):73–165 (discussion 306–7).
Dong XS. The malaria vectors and their ecology in Yunnan Province. Chin J Parasit Dis. 2000;13:144–7 (in Chinese).
Yang HL, Zhou HN. Yunnan malaria. Kunming: Yunnan Science and Technology Press; 2015. (in Chinese).
Xu JW, Liu H, Yaw B, Nbwi HS. The health beliefs, dengue knowledge and control behaviors among internally displaced persons versus local residents in Kachin Special Region II, Myanmar. PLoS Negl Trop Dis. 2020;14:e0008321.
Xu JW, Li Y, Yang HL, Zhang J, Zhang ZX, Yang YM, et al. Malaria control along China–Myanmar Border during 2007–2013: an integrated impact evaluation. Infect Dis Poverty. 2016;5:75.
Xu JW, Li JJ, Guo HP, Pu SW, Li SM, Wang RH, et al. Malaria from hyperendemicity to elimination in Hekou County on China–Vietnam border: an ecological study. Malar J. 2017;16:66.
WHO. Malaria elimination: a field manual for low and moderate endemic countries. Geneva: World Health Organization; 2014.
Liu H, Xu JW, Bi Y. Malaria burden and treatment targets in Kachin Special Region II, Myanmar from 2008 to 2016: a retrospective analysis. PLoS ONE. 2018;13:e0195032.
Xu JW, Wang WR, Xu SY. Malaria situation and control in border areas and Yuanjiang River Basin of Yunnan Province in 1995. Chin J Parasit Dis. 1998;11(1):71 (in Chinese).
China MOH, Myanmar MOHS. The agreement of malaria control in China–Myanmar border areas. Kunming: China ministry of health, Ministry of Health and Sports of Myanmar. 2005. http://106.58.220.4:8010/eofce10/client/web/login. Accessed 9 June 2021.
Zhou H, Du L, Yang H, Zhang Z. Innovative decade of cross-border joint prevention & control project of malaria and dengue fever in Yunnan China-GMS areas. Kunming: Yunnan Science and Technology Press; 2015.
Xu JW, Lee R, Li XH, Liu H. Transition of radical, preventive and presumptive treatment regimens for malaria in China: a systematic review. Malar J. 2021;20:10. https://doi.org/10.1186/s12936-020-03535-8.
Zhang ZX, Zhou S, Xu JW, Du ZW, Chen GW, Li L, et al. Investiagtion on missing-report of malaria cases in Yunnan, China. Chin J Parasit Dis. 2004;17(1):25–7 (in Chinese).
Cao J, Sturrock HJ, Cotter C, Zhou S, Zhou H, Liu Y, et al. Communicating and monitoring surveillance and response activities for malaria elimination: China’s ‘“1-3-7”’ Strategy. PLoS Med. 2014;11(5):e1001642.
Ministry of Health of the People’s Republic of China. Action plan of China malaria elimination (2010–2020). 2012. http://www.gov.cn/gzdt/att/att/site1/20100526/001e3741a2cc0d67233801.doc. Accessed 15 Oct 2020.
China Yunnan Health Department. The protocol of Yunnan malaria elimination action plan (2010–2020). Kunming: Yunnan Health Department; 2010. Accessed 27 Feb 2019. (in Chinese)
Zhao X, Sun X, Yang H, Zhou D, Yang J, Guo T, et al. A report of the last indigenous malaria case in Yunnan. Chin Trop Med. 2020;20:325–8 (in Chinese).
WHO. A framework for malaria elimination. Geneva: World Health Organization; 2017.
Guerra AC, Reiner JRR, Perkins TA, Lindsay SW, Midega JT, Brady O, et al. A global assembly of adult female mosquito mark-release-recapture data to inform the control of mosquito-borne pathogens. Parasit Vectors. 2014;7:276.
Thanh PV, Hong NV, Van NV, Malderen CV, Obsomer V, Urgell AR, et al. Epidemiology of forest malaria in Central Vietnam: the hidden parasite reservoir. Malar J. 2015;14:86.
Kounnavong S, Gopinath D, Hongvanthong B, Khamkong C, Sichanthongthip O. Malaria elimination in Lao PDR: the challenges associated with population mobility. Infect Dis Poverty. 2017;6:81.
Song J, Socheat D, Tan B, Dara P, Deng C, Sreng Sokunthea S, et al. Rapid and effective malaria control in Cambodia through mass administration of artemisinin-piperaquine. Malar J. 2010;9:57.
Wangdi K, Furuya-Kanamori L, Clark J, Barendregt JJ, Gatton ML, Banwell C, et al. Comparative effectiveness of malaria prevention measures: a systematic review and network meta-analysis. Parasit Vectors. 2018;11:210.
Huber JH, Koepfli C, España G, Nekkab N, White MT, Alex PT. How radical is radical cure? Site-specific biases in clinical trials underestimate the effect of radical cure on Plasmodium vivax hypnozoites. Malar J. 2021;20(1):479.
Liu H, Xu JW, Yang HL, Li M, Sun CD, Yin YJ, et al. Investigation and control of a Plasmodium falciparum malaria outbreak in Shan Special Region II of Myanmar along the China-Myanmar Border from June to December 2014. Infect Dis Poverty. 2016;5:32.
Acknowledgements
We would like to thank Dr. Hai-Mo Shen from the Chinese Center for Disease Control and Prevention, National Institute of Parasitic Diseases for creating the figures.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81560543 and 81673113).
Author information
Authors and Affiliations
Contributions
HNZ, JWX and HL conceived the work. JWX and HL collected data and wrote the manuscript. YWZ, YD, ZRL, CLZ, QYC, CW, KXD and PT provided critical comments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Supplementary Information
Additional file 1: Table S1.
Malaria area stratification and interventions in border areas, Yunnan, 2003‒2013. Table S2. The annual coverage of laboratory tests for malaria parasites and preventive treatment in the Yunnan border area, 2003‒2020. Table S3. The annual parasite incidence (API) in the Yunnan border area, 2003‒2013. Table S4. The number of malaria cases detected and the categories in the Yunnan border area, 2014‒2020. Table S5. Annual parasite incidence (API) in 25 border counties, Yunnan 2003, 2006, 2010 and 2013. Table S6. Malaria cases detected and categories in 25 border counties, Yunnan, 2014‒2020. Table S7. High risk villages of imported malaria by parasite-infected anophelines in 2018.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, H., Zhou, Y., Deng, Y. et al. Malaria from hyperendemicity to elimination along international borders in Yunnan, China during 2003‒2020: a case study. Infect Dis Poverty 11, 51 (2022). https://doi.org/10.1186/s40249-022-00972-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40249-022-00972-2